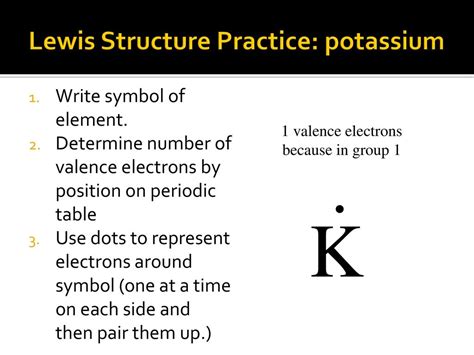
lewis structure potassium|Oops. Something went wrong. Please try again. : Manila A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. Make your fantasies come true with Pleasures.ai, the most realistic NSFW AI image generator. Unlimited, uncensored, and easy to use.
PH0 · Oops. Something went wrong. Please try again.
PH1 · Lewis Structure Finder
PH2 · Lewis Electron Dot Structures
PH3 · Lewis Dot Structure for Potassium
PH4 · How to draw the K+ Lewis Dot Structure
PH5 · Drawing Lewis Structures
PH6 · 9.3: Drawing Lewis Structures
PH7 · 9.2: Lewis Electron Dot Diagrams
PH8 · 7.3 Lewis Symbols and Structures
PH9 · 5.3: Lewis Diagrams
Аппаратный сброс SAMSUNG Galaxy A12. Как сбросить установки до . Hard Reset SAMSUNG Galaxy A12 – Bypass Screen Lock / Factory Reset by Recovery Mode - видео .
lewis structure potassium*******Figure 5.3.1 5.3. 1 The figure above shows the electron shells of He (Helium), Cl (Chlorine), and K (Potassium) as well as their Lewis dot structures below. Notice how both . For the K+ structure use the periodic table to find the total number of valence electrons for K. Once we know how many valence electrons there are in Potassi. A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule.
lewis structure potassiumGet the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around .Watch this video to learn how to draw Lewis diagrams for molecules and ions, using the octet rule and formal charges.This free textbook is an OpenStax resource written to increase student access to high-quality, peer-reviewed learning materials.Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the .A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. The Lewis structure was named after Gilbert N. Lewis, who introduced it in his 1916 article The Atom and the Molecule. Three potassium atoms lose one electron each, to become three +1 ions (cations).One nitrogen atom gains three electrons to become a -3 ion (anion).These are . Potassium, a metal in Group 1, loses one electron to become a +1 ionIodine, a non-metal in Group 17, gains one electron to become a -1 ion.Together, they com. The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. Chemists usually indicate a bonding pair by a single .When the Lewis structure of an ion is written, the entire structure is placed in brackets, and the charge is written as a superscript on the upper right, outside of the brackets. For example, consider the ammonium ion, NH 4 + , which contains 9 (5 from N and 1 from each of the four H atoms) –1 = 8 electrons.
The representation of molecules in Lewis electron dot structure or just a Lewis structure is in honour of the American chemist Gilbert Newton Lewis. Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in . Potassium, a metal in Group 1, loses one electron to become a +1 ionBromine, a non-metal in Group 17, gains one electron to become a -1 ion.Together, they co.lewis structure potassium Oops. Something went wrong. Please try again. Hence, the final Bohr model of the Potassium atom consists of 19 protons, 20 neutrons, and 19 electrons. The electrons are housed in four shells i.e. K, L, M, and N shells as 2, 8, 8, and 1, respectively. Deriving Lewis Structure from Bohr Model. Both Lewis structure and Bohr model of an atom describes its structure through visual representation.
A Lewis electron dot symbol (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol . A step-by-step explanation of how to draw the K2CO3 Lewis Dot Structure.For K2CO3 we have an ionic compound and we need to take that into account when we dra.
Oops. Something went wrong. Please try again. Potassium, a metal in Group 1, loses one electron to become a +1 ionFluorine, a non-metal in Group 17, gains one electron to become a -1 ion.Together, they c.

Drawing a probable Lewis Structure gives us a clear picture of a 2D molecular composition along with electron dots and bond formation. Despite its few limitations, the initial step towards deciphering chemical bonding is via . A step-by-step explanation of how to draw the K2S Lewis Dot Structure.For K2S we have an ionic compound and we need to take that into account when we draw th.
The Lewis structure of KCl, or potassium chloride, is an essential concept in understanding the electron arrangement and bonding in chemical compounds.By following a series of steps, we can determine the Lewis structure of KCl, which provides valuable insights into its properties and behavior.. Step 1: Counting Valence Electrons. To begin, we need to . A step-by-step explanation of how to draw the KF Lewis Dot Structure.For KF we have an ionic compound and we need to take that into account when we draw the .
The potassium nitride lewis structure involves the transfer of an electron from potassium to nitrogen, resulting in the formation of an ionic bond. The potassium atom loses an electron to form a positively charged ion (K+), while the nitrogen atom gains an electron to form a negatively charged ion (N-). The resulting compound has a net charge .
The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). A dash (or line) is usually used to indicate a shared pair of electrons: In the Lewis model, a single shared pair of electrons is a single bond. Each Cl atom . Potassium sulfide (K2S) consists of two potassium (K) atoms, each with 1 valence electron, and a sulfur (S) atom with 6 valence electrons. In the Lewis structure, each K atom donates its electron to S, forming ionic bonds and resulting in a K+ ion and an S2- ion.
Potassium perchlorate can be used as an antithyroid agent used to treat hyperthyroidism, usually in combination with one other medication.This application exploits the similar ionic radius and hydrophilicity of perchlorate and iodide.. The administration of known goitrogen substances can also be used as a prevention in reducing the biological uptake of iodine, (whether it is the .
Get the latest horse racing results from Racenet with TAB dividends including exotics. Find out all the latest tab horse racing results for today and the past meetings by tracks, horses, jockeys, trainers, states and countries.
lewis structure potassium|Oops. Something went wrong. Please try again.